Quality and Regulatory Compliance
Proven quality & regulatory compliance
You need to bring superior medical innovations to market efficiently, which means there is no room for error when it comes to patient safety and product efficacy. Ascential™ is a trusted partner committed to excellence with every product, process, and service. We help you monitor quality controls and compliance from design through launch so that you can improve lives across the world safely and rapidly.
Get Your 15-Minute Consultation
Trusted by leading brands













































Ascential™ Quality Statement
Ascential Medical and Life Sciences™ is deeply committed to advancing healthcare and improving patient outcomes through innovation in science and technology. This includes delivering products and services that meet the highest standards of quality. Our commitment is embedded in every aspect of our operations across more than 40 facilities worldwide.
Ascential’s state-of-the-art facilities maintain rigorous quality standards that are regularly evaluated through internal audits, regulatory reviews, and customer feedback to ensure continuous improvement and compliance.
Our mission is to support our customers in bringing superior medical innovations to market efficiently. By placing patient safety and product efficacy at the forefront of our priorities, we aim to improve health outcomes and drive industry standards forward. Our team works to foster industry-leading practices, deliver exceptional value to our customers, and improve lives worldwide.
We understand the responsibility that comes with manufacturing and developing medical products, and we are committed to upholding the trust placed in us by our customers, clinicians, and their patients. Ascential is your trusted partner, committed to excellence in every product and process we design.

Certifications
| Country | Location | Division Name | ISO 9001 | ISO 14001 | ISO 13485 | FDA Registered | California Medical Device Manufacturing Licensed |
|---|---|---|---|---|---|---|---|
| USA | Blaine, MN | Aspect Automation, LLC | Download | ||||
| USA | San Diego, CA | D & K Engineering | Download | Download #MD793157 |
#3005268307 21 CFR Part 820 & Part 11 Compliant |
Download #121465 |
|
| USA | Ham Lake, MN | NACS Inc. | Download | Download | |||
| USA | Corvallis, OR | Korvis LLC | Download | Download | |||
| Singapore | Singapore | Ascential Singapore | Download | Download | |||
| Malaysia | Malaysia | Ascential Malaysia | Download | Download | Download |
| Certification/Compliance | Details |
|---|---|
| FDA Registered | FEI Number 3011026927, Establishment Registration & Device Listing |
| Minnesota Board of Pharmacy | Details at MN Health Board (Search License #461991) or Download |
| 21 CFR 210/211 Compliant | Details - Part 210, Details - Part 211 |
| 21 CFR 820 Compliant | Details |
| 21 CFR Part 4 Compliant | Details |
| 21 CFR Part 11 Compliant | Details |
| State of California Department of Public Health Medical Device Manufacturing License | Download |
Services
Quality controls built for medical innovations
Quality Management & Training Systems
Strengthen operational clarity and team performance through comprehensive resource management.
Documentation Remediation
Identify and resolve any design and/or documentation issues inherited from other suppliers.
Supplier & Purchasing Quality Assurance
Ensure only trusted, compliant components enter your production process.
Purchasing, Design, & Production Controls
Manufacture precisely-engineered products with integrated oversight of controls. Centrally manage all plans, tests, verifications, and inspections.
Infrastructure & Equipment Controls
Implement consistent safety, materials, warehouse, and facility controls (including pest control). Our experts handle equipment qualification, calibration, and preventative maintenance.
Capabilities
Comprehensive medical device manufacturing & regulatory expertise
Lean Manufacturing Principles
Minimize production waste and streamline all processes with the Ascential Production System (APS).
Robust Quality Management System (QMS)
Operate with confidence under a robust QMS registered and certified by key regulatory bodies.
Continuous Quality Improvement
Maintain rigorous quality standards that are regularly evaluated through internal audits, regulatory reviews, and customer feedback.

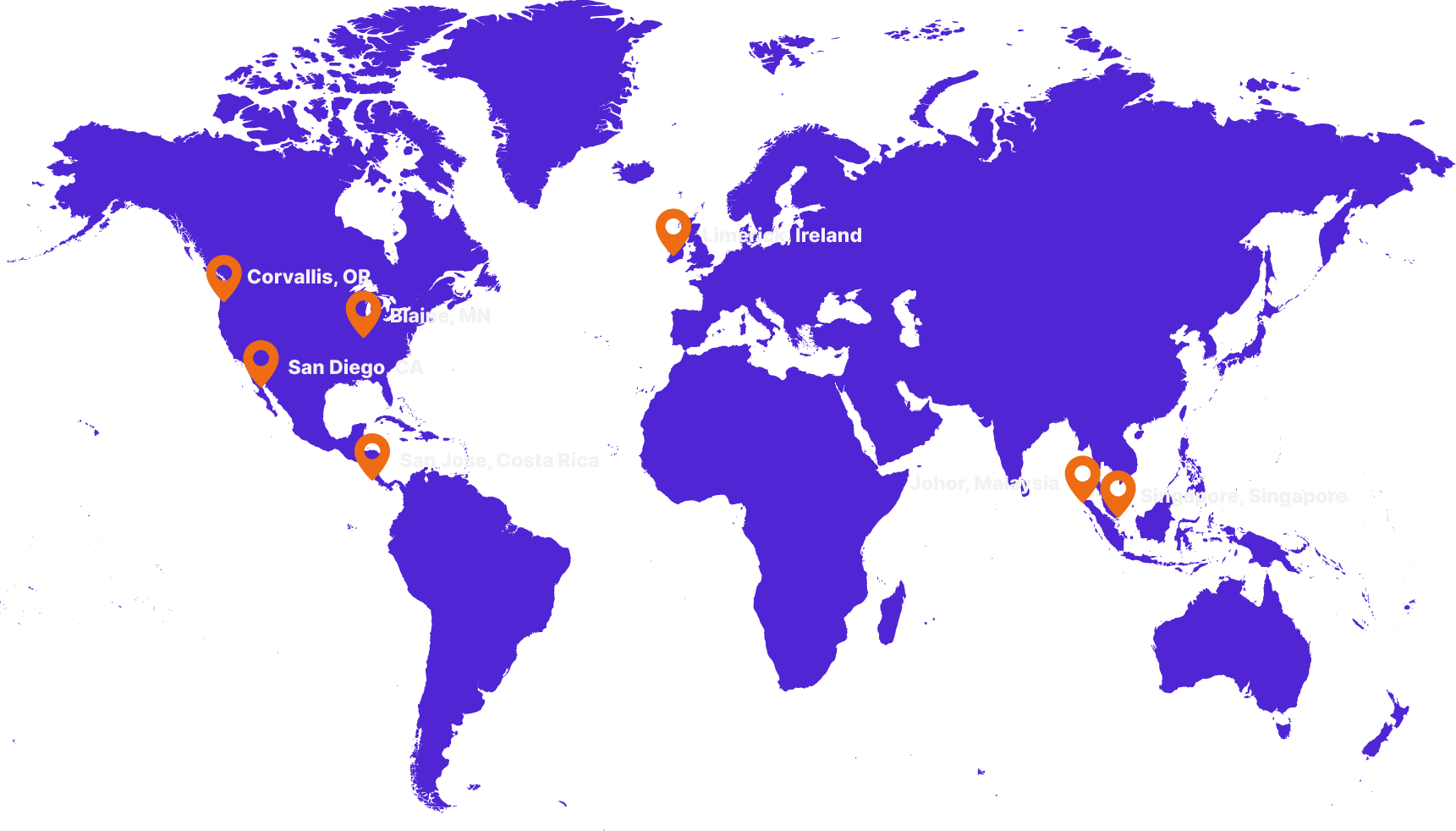
Medical manufacturing capabilities
around the world.
We’re well-positioned to deliver comprehensive, scalable solutions for your design, automation, and manufacturing challenges anywhere in the world. Our commitment to quality is embedded in every aspect of our operations across more than 40 facilities across the globe:
Frequently Asked Questions
Get answers to common questions about our quality & regulatory compliance capabilities.
We implement lean manufacturing principles across the board and rely on the following systems to help you make more informed decisions about quality control and management for your medical manufacturing processes:
- Continuous regulatory compliance improvement of the Quality Management System, including implementation of EU IVDR 2017/746 and EU MDR 2017/746
- QMS 2.0 Quality Plan (D011251)
- Industry-leading eQMS (ETQ Reliance)
- eQMS Implementation Quality Plan (D013174)
- A new state-of-the-art Manufacturing Execution System (MES-Visual Factory)
- MES Implementation Quality Plan (D013458)
- Management Responsibility & Commitment
- Quality Manual & Policy
- Management Reviews
- Resource Management
- Training
- Certifications
- Performance Evaluations
- Documentation Requirements
- Change Control
- Control of Records
- Periodic Reviews
- Purchasing Controls
- Supplier Qualifications
- Supplier Monitoring & Corrective Actions
- Part Qualifications
- Materials Compliance (Reach, RoHS, Conflict Minerals, etc.)
- Receiving Inspection
- Design Controls
- Design & Development Planning
- Design & Development Input
- Design & Development Output
- Design Reviews
- Design Verification
- Design Transfer
- Risk Management
- Design History File (DHF)
- Production Controls
- Manufacturing Process Validations
- Work & Test Instructions
- Label Control
- End of Line Testing
- In-Process & Final Quality Inspections
- Quality Release
- Device History Records (DHR)
- Device Master Record (DMR)
- Internal Audits
- Annual ISO Surveillance Audits
- Quarterly NRTL Audits
- Customer Feedback
- Customer Complaints/SCARs
- Planned Deviations
- NCRs
- CAPAs
Yes. Through our global network of FDA/ISO-certified facilities, Ascential mitigates commercialization risks with thorough design and documentation remediation, robust quality systems, and cross-functional collaboration across engineering, manufacturing, supply chain, and regulatory teams. Our experience in problem-solving, root-cause analysis, and regulatory navigation helps clients bring products to market faster and with confidence.
Ascential’s experienced quality team, drawn from leading medical device companies, leverages deep regulatory, quality, and operational expertise to ensure accurate documentation and smooth commercialization. While we reduce risks related to manufacturing quality and regulatory compliance, you can feel empowered to focus on your innovation.
We have a proven record of no recalls, FDA 483s, or consent decrees. Our team of engineers and quality experts brings decades of experience in product development, design transfer, regulatory compliance, and manufacturing.
Reduce risk. Avoid delays. Protect brand integrity.
Learn how Ascential can bring your innovative medical devices and instruments to market faster and help you contain costs with proven quality and regulatory compliance services.
Get Your 15-Minute Consultation
