Scale where
you need it
Bridge development gaps across engineering, automation, and manufacturing with Ascential™. Add scale where you need it and momentum when it matters.
Get Your 15-Minute Consultation
Product Development
Turn brilliant ideas into regulated reality
Translate concepts into products
that withstand regulatory review.
Advanced Manufacturing
Scale complex
production safely
Manufacture globally without revalidating or
losing momentum.
Automation
Treat process like
its own product
Streamline production with workflows
engineered as precisely as your device.
7 legacies.
One partner.
Ascential brings together the expertise of seven well-respected, highly specialized firms across engineering, automation, and advanced manufacturing. United, we help the world’s leading medical device and life sciences companies solve their most critical manufacturing and design challenges.

Trusted by innovators & industry leaders
From startups fast-tracking a breakthrough to OEMs expanding globally, Ascential is trusted for when complexity peaks, timelines slip, and momentum stalls.













































Case Studies
On time. In full. With polish.
Explore in-depth case studies on our work. With Ascential, every quote, prototype, validation, build, and transfer is delivered completely and correctly to spec. No delays. Never halfway.
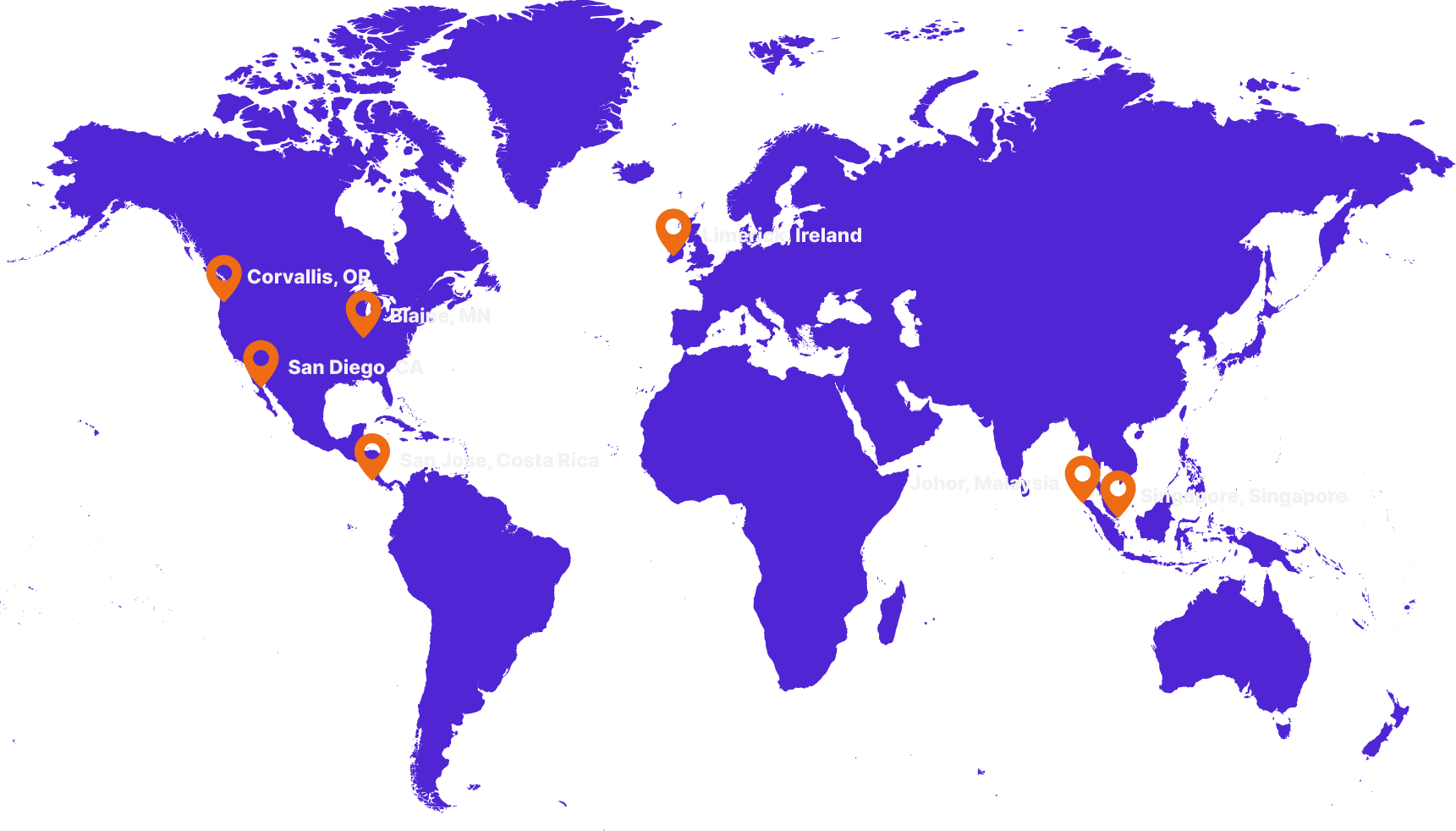
Scale here. Build there.
Compliant everywhere.
Scale easier with our global network of harmonized FDA/ISO-certified facilities. Prototype in the U.S. and build in Europe or Costa Rica, all without revalidation. Wherever production happens, the same systems, standards, and oversight follow.
Let’s solve your engineering challenge
Explore how Ascential can fit into your process and turn your advanced device concept into scalable, market-ready products.
Get Your 15-Minute Consultation



